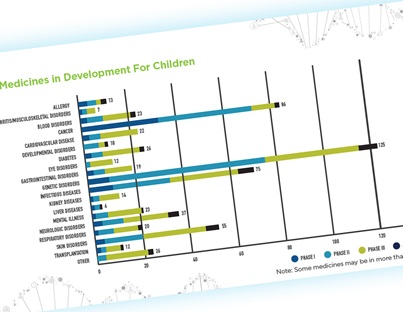
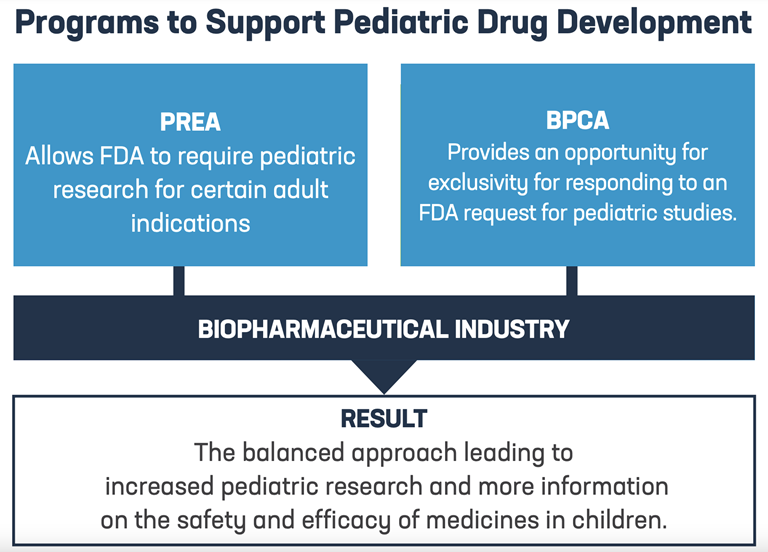
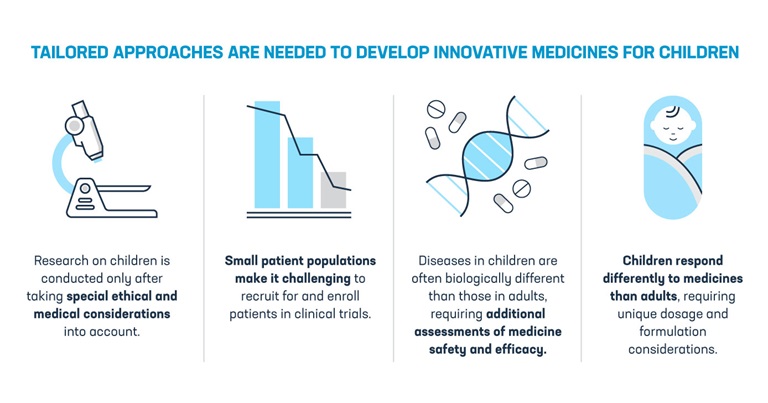
Manage My Cookies
Your privacy is important to us. Cookies are very small text files that are stored on your computer when you visit a website. We use cookies and other tracking technologies for a variety of purposes and to enhance your online experience on our website. You can change your preferences and decline certain types of cookies to be stored on your computer while browsing our website. You can also remove any cookies already stored on your computer, but keep in mind that deleting cookies may prevent you from using parts of our website. You can check our privacy policy page for more information.
-
Always ActiveThese cookies are essential to provide you with services available through our website and to enable you to use certain features of our website. Without these cookies, we cannot provide you certain services on our website; as a result these cookies do not require your consent.These cookies are used to show advertising that is likely to be of interest to you based on your browsing habits. These cookies, as served by our content and/or advertising providers, may combine information they collected from our website with other information they have independently collected relating to your web browser's activities across their network of websites.These cookies are used to provide you with a more personalized experience on our website and to remember choices you make when you use our website. For example, we may use functionality cookies to remember your language preferences or remember your login details.These cookies are used to collect information to analyze the traffic to our website and how visitors are using our website. For example, these cookies may track things such as how long you spend on the website or the pages you visit which helps us to understand how we can improve our website site for you.Always ActiveThese cookies are essential to provide you with services available through our website and to enable you to use certain features of our website. Without these cookies, we cannot provide you certain services on our website; as a result these cookies do not require your consent.These cookies are used to show advertising that is likely to be of interest to you based on your browsing habits. These cookies, as served by our content and/or advertising providers, may combine information they collected from our website with other information they have independently collected relating to your web browser's activities across their network of websites.These cookies are used to provide you with a more personalized experience on our website and to remember choices you make when you use our website. For example, we may use functionality cookies to remember your language preferences or remember your login details.These cookies are used to collect information to analyze the traffic to our website and how visitors are using our website. For example, these cookies may track things such as how long you spend on the website or the pages you visit which helps us to understand how we can improve our website site for you.